Publications

A Genetically-Defined Circuit for Arousal from Sleep during Hypercapnia
The precise neural circuitry that mediates arousal during sleep apnea is not known. We previously found that glutamatergic neurons in the external lateral parabrachial nucleus (PBel) play a critical role in arousal to elevated CO2 or hypoxia. Because many of the PBel neurons that respond to CO2 express calcitonin gene-related peptide (CGRP), we hypothesized that CGRP may provide a molecular identifier of the CO2 arousal circuit. Here we report that selective chemogenetic and optogenetic activation of PBelCGRP neurons caused wakefulness, whereas optogenetic inhibition of PBelCGRP neurons prevented arousal to CO2, but not to an acoustic tone or shaking. Optogenetic inhibition of PBelCGRP terminals identified a network of forebrain sites under the control of a PBelCGRP switch that is necessary to arouse animals from hypercapnia. Our findings define a novel cellular target for interventions that may prevent sleep fragmentation and the attendant cardiovascular and cognitive consequences seen in obstructive sleep apnea.

Targeted disruption of supraspinal motor circuitry reveals a distributed network underlying Restless Legs Syndrome (RLS)-like movements in the rat
In this study we uncovered, through targeted ablation, a potential role for corticospinal, cerebello-rubro-spinal, and hypothalamic A11 dopaminergic systems in the development of restless legs syndrome (RLS)-like movements during sleep. Targeted lesions in select basal ganglia (BG) structures also revealed a major role for nigrostriatal dopamine, the striatum, and the external globus pallidus (GPe) in regulating RLS-like movements, in particular pallidocortical projections from the GPe to the motor cortex. We further showed that pramipexiole, a dopamine agonist used to treat human RLS, reduced RLS-like movements. Taken together, our data show that BG-cortico-spinal, cerebello-rubro-spinal and A11 descending projections all contribute to the suppression of motor activity during sleep and sleep-wake transitions, and that disruption of these circuit nodes produces RLS-like movements. Taken together with findings from recent genomic studies in humans, our findings provide additional support for the concept that the anatomic and genetic etiological bases of RLS are diverse.

Neurotensin Receptor-1 Identifies a Subset of Ventral Tegmental Dopamine Neurons that Coordinates Energy Balance
Dopamine (DA) neurons in the ventral segmental area (VTA) are heterogeneous and differentially regulate ingestive and locomotor behaviors that affect energy balance. Identification of which VTA DA neurons mediate behaviors that limit weight gain has been hindered, however, by the lack of molecular markers to distinguish VTA DA populations. Here, we identified a specific subset of VTA DA neurons that express neurotensin receptor-1 (NtsR1) and preferentially comprise mesolimbic, but not mesocortical, DA neurons. Genetically targeted ablation of VTA NtsR1 neurons uncouples motivated feeding and physical activity, biasing behavior toward energy expenditure and protecting mice from age-related and diet-induced weight gain. VTA NtsR1 neurons thus represent a molecularly defined subset of DA neurons that are essential for the coordination of energy balance. Modulation of VTA NtsR1 neurons may therefore be useful to promote behaviors that prevent the development of obesity.

Carbon Monoxide Preserves Circadian Rhythm to Reduce the Severity of Subarachnoid Hemorrhage in Mice
Subarachnoid hemorrhage (SAH) is associated with a temporal pattern of stroke incidence. We hypothesized that natural oscillations in gene expression controlling circadian rhythm impact the severity of neuronal injury. We moreover predict that heme oxygenase-1 (HO-1/Hmox1) and its product carbon monoxide (CO) contribute to restoration of rhythm and neuroprotection.

Activation of the GABAergic Parafacial Zone Maintains Sleep and Counteracts the Wake-Promoting Action of the Psychostimulants Armodafinil and Caffeine
We previously reported that acute and selective activation of GABA-releasing parafacial zone (PZVgat) neurons in behaving mice produces slow-wave-sleep (SWS), even in the absence of sleep deficit, suggesting that these neurons may represent, at least in part, a key cellular substrate underlying sleep drive. It remains, however, to be determined if PZVgat neurons actively maintain, as oppose to simply gate, SWS. To begin to experimentally address this knowledge gap, we asked whether activation of PZVgat neurons could attenuate or block the wake-promoting effects of two widely used wake-promoting psychostimulants, armodafinil or caffeine. We found that activation of PZVgat neurons completely blocked the behavioral and electrocortical wake-promoting action of armodafinil. In some contrast, activation of PZVgat neurons inhibited the behavioral, but not electrocortical, arousal response to caffeine. These results suggest that: (1) PZVgat neurons actively maintain, as oppose to simply gate, SWS and cortical slow-wave-activity; (2) armodafinil cannot exert its wake-promoting effects when PZVgat neurons are activated, intimating a possible shared circuit/molecular basis for mechanism of action; (3) caffeine can continue to exert potent cortical desynchronizing, but not behavioral, effects when PZVgat neurons are activated, inferring a shared and divergent circuit/molecular basis for mechanism of action; and 4) PZVgat neurons represent a key cell population for SWS induction and maintenance.

Catecholaminergic A1/C1 Neurons Contribute to the Maintenance of Upper Airway Muscle Tone but may not Participate in NREM Sleep-Related Depression of these Muscles
Neural mechanisms of obstructive sleep apnea, a common sleep-related breathing disorder, are incompletely understood. Hypoglossal motoneurons, which provide tonic and inspiratory activation of genioglossus (GG) muscle (a major upper airway dilator), receive catecholaminergic input from medullary A1/C1 neurons. We aimed to determine the contribution of A1/C1 neurons in control of GG muscle during sleep and wakefulness. To do so, we placed injections of a viral vector into DBH-cre mice to selectively express the hMD4i inhibitory chemoreceptors in A1/C1 neurons. Administration of the hM4Di ligand, clozapine-N-oxide (CNO), in these mice decreased GG muscle activity during NREM sleep (F1,1,3=17.1, p<0.05); a similar non-significant decrease was observed during wakefulness. CNO administration had no effect on neck muscle activity, respiratory parameters or state durations. In addition, CNO-induced inhibition of A1/C1 neurons did not alter the magnitude of the naturally occurring depression of GG activity during transitions from wakefulness to NREM sleep. These findings suggest that A1/C1 neurons have a net excitatory effect on GG activity that is most likely mediated by hypoglossal motoneurons. However, the activity of A1/C1 neurons does not appear to contribute to NREM sleep-related inhibition of GG muscle activity, suggesting that A1/C1 neurons regulate upper airway patency in a state-independent manner.

Wake-Sleep Circuitry: An Overview
Although earlier models of brain circuitry controlling wake-sleep focused on monaminergic and cholinergic arousal systems, recent evidence indicates that these play mainly a modulatory role, and that the backbone of the wake-sleep regulatory system depends upon fast neurotransmitters, such as glutmate and GABA. We review here recent advances in understanding the role these systems play in controlling sleep and wakefulness.
Brainstem regulation of slow-wave-sleep
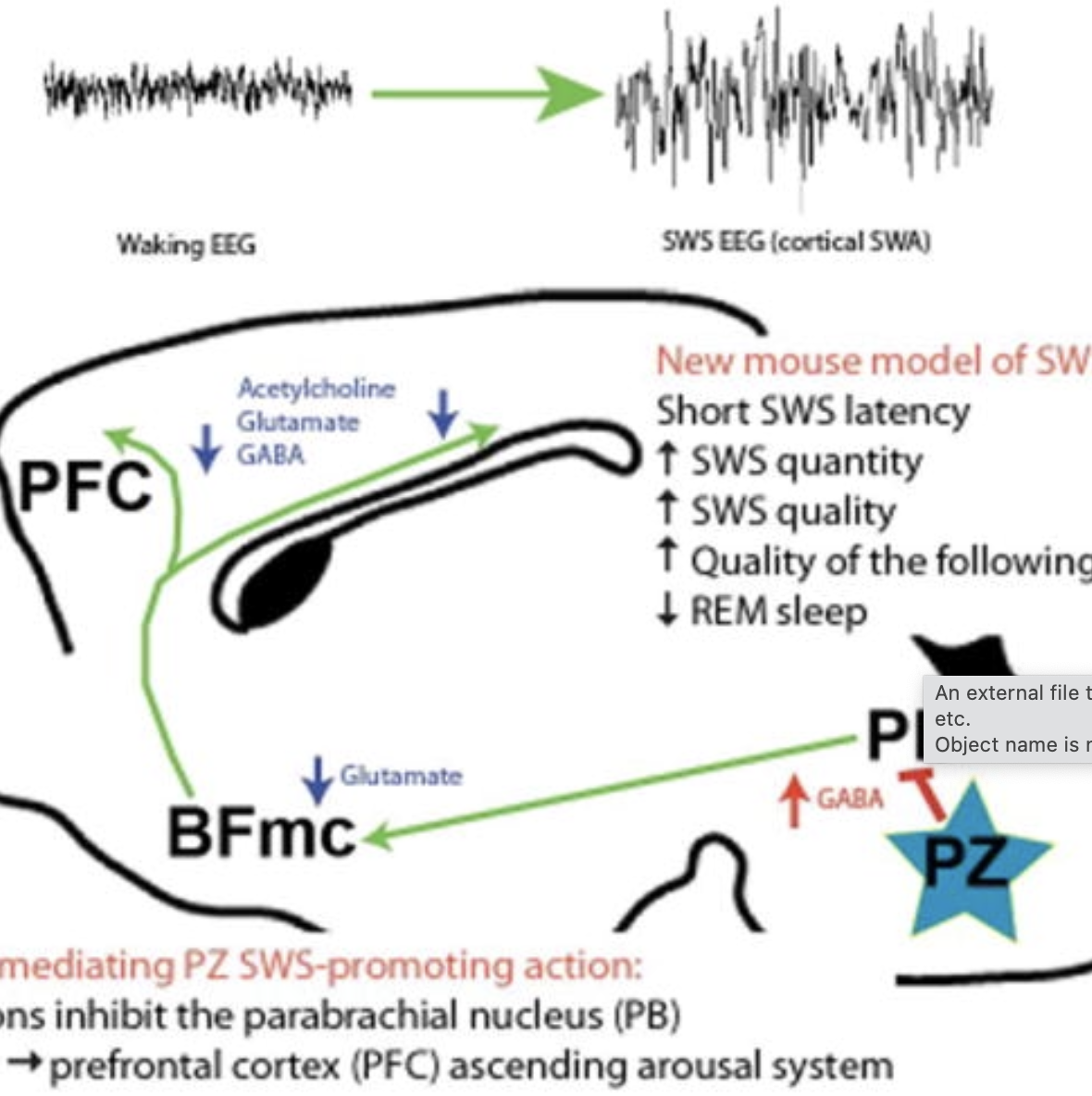
Recent work has helped reconcile puzzling results from brainstem transection studies first performed over 60 years ago, which suggested the existence of a sleep-promoting system in the medullary brainstem. It was specifically shown that GABAergic neurons located in the medullary brainstem parafacial zone (PZGABA) are not only necessary for normal slow-wave-sleep (SWS) but that their selective activation is sufficient to induce SWS in behaving animals. In this review we discuss early experimental findings that inspired the hypothesis that the caudal brainstem contained SWS-promoting circuitry. We then describe the discovery of the SWS-promoting PZGABA and discuss future experimental priorities.
Ventral medullary control of rapid eye movement sleep and atonia
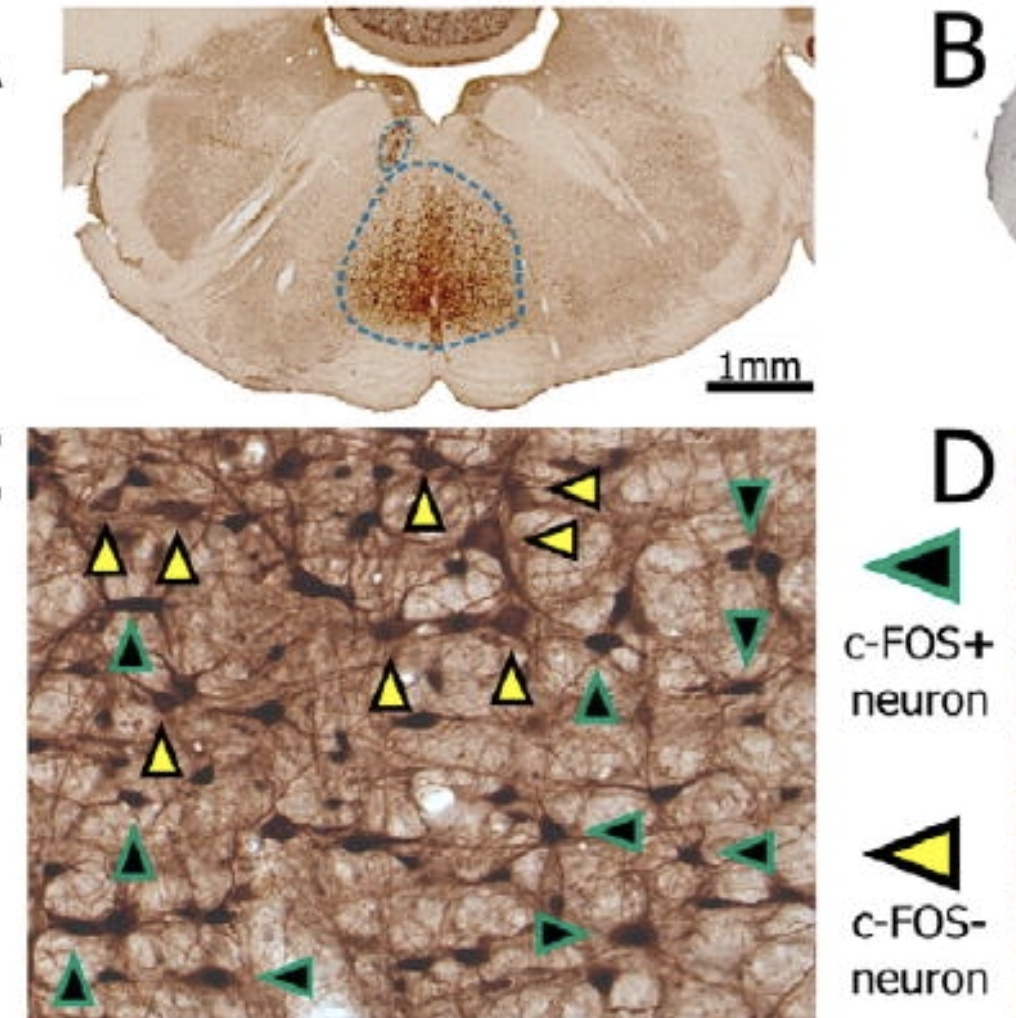
Discrete populations of neurons at multiple levels of the brainstem control rapid eye movement (REM) sleep and the accompanying loss of postural muscle tone, or atonia. The specific contributions of these brainstem cell populations to REM sleep control remains incompletely understood. Here we show in rats that viral vector-based lesions of the ventromedial medulla at a level rostral to the inferior olive (pSOM) produced violent myoclonic twitches and abnormal electromyographic spikes, but not complete loss of tonic atonia, during REM sleep. Motor tone during non-REM (NREM) sleep was unaffected in these same animals. Acute chemogenetic activation of pSOM neurons in rats robustly and selectively suppressed REM sleep but not NREM sleep. Similar lesions targeting the more rostral ventromedial medulla (RVM) did not affect sleep or atonia, while chemogenetic stimulation of the RVM produced wakefulness and reduced sleep. Finally, selective activation of vesicular GABA transporter (VGAT) pSOM neurons in mice produced complete suppression of REM sleep whereas their loss increased EMG spikes during REM sleep. These results reveal a key contribution of the pSOM and specifically the VGAT+ neurons in this region in REM sleep and motor control.

Cholinergic, Glutamatergic, and GABAergic Neurons of the Pedunculopontine Tegmental Nucleus Have Distinct Effects on Sleep/Wake Behavior in Mice
The pedunculopontine tegmental (PPT) nucleus has long been implicated in the regulation of cortical activity and behavioral states, including rapid eye-movement (REM) sleep. For example, electrical stimulation of the PPT region during sleep leads to rapid awakening, whereas lesions of the PPT in cats reduce REM sleep. Though these effects have been linked with the activity of cholinergic PPT neurons, the PPT also includes intermingled glutamatergic and GABAergic cell populations, and the precise roles of cholinergic, glutamatergic, and GABAergic PPT cell groups in regulating cortical activity and behavioral state remain unknown. Using a chemogenetic approach in three Cre-driver mouse lines, we found that selective activation of glutamatergic PPT neurons induced prolonged cortical activation and behavioral wakefulness, whereas inhibition reduced wakefulness and increased non-REM (NREM) sleep. Activation of cholinergic PPT neurons suppressed lower-frequency electroencephalogram rhythms during NREM sleep. Last, activation of GABAergic PPT neurons slightly reduced REM sleep. These findings reveal that glutamatergic, cholinergic, and GABAergic PPT neurons differentially influence cortical activity and sleep/wake states.

A Novel Population of Wake-Promoting GABAergic Neurons in the Ventral Lateral Hypothalamus
The largest synaptic input to the sleep-promoting ventrolateral preoptic area (VLPO) arises from the lateral hypothalamus, a brain area associated with arousal. However, the neurochemical identity of the majority of these VLPO-projecting neurons within the lateral hypothalamus (LH), as well as their function in the arousal network, remains unknown. Herein we describe a population of VLPO-projecting neurons in the LH that express the vesicular GABA transporter (VGAT; a marker for GABA-releasing neurons). In addition to the VLPO, these neurons also project to several other established sleep and arousal nodes, including the tuberomammillary nucleus, ventral periaqueductal gray, and locus coeruleus. Selective and acute chemogenetic activation of LH VGAT+ neurons was profoundly wake promoting, whereas acute inhibition increased sleep. Because of its direct and massive inputs to the VLPO, this population may play a particularly important role in sleep-wake switching.
How genetically engineered systems are helping to define, and in some cases redefine, the neurobiological basis of sleep and wake
The advent of genetically engineered systems, including transgenic animals and recombinant viral vectors, has facilitated a more detailed understanding of the molecular and cellular substrates regulating brain function. In this review we highlight some of the most recent molecular biology and genetic technologies in the experimental “systems neurosciences,” many of which are rapidly becoming a methodological standard, and focus in particular on those tools and techniques that permit the reversible and cell-type specific manipulation of neurons in behaving animals. These newer techniques encompass a wide range of approaches including conditional deletion of genes based on Cre/loxP technology, gene silencing using RNA interference, cell-type specific mapping or ablation and reversible manipulation (silencing and activation) of neurons in vivo. Combining these approaches with viral vector delivery systems, in particular adeno-associated viruses (AAV), has extended, in some instances greatly, the utility of these tools. For example, the spatially- and/or temporally-restricted transduction of specific neuronal cell populations is now routinely achieved using the combination of Cre-driver mice and stereotaxic-based delivery of AAV expressing Cre-dependent cassettes. We predict that the experimental application of these tools, including creative combinatorial approaches and the development of even newer reagents, will prove necessary for a complete understanding of the neuronal circuits subserving most neurobiological functions, including the regulation of sleep and wake.

The anatomical, cellular and synaptic basis of motor atonia during rapid eye movement sleep
Rapid eye movement (REM) sleep is a recurring part of the sleep–wake cycle characterized by fast, desynchronized rhythms in the electroencephalogram (EEG), hippocampal theta activity, rapid eye movements, autonomic activation and loss of postural muscle tone (atonia). The brain circuitry governing REM sleep is located in the pontine and medullary brainstem and includes ascending and descending projections that regulate the EEG and motor components of REM sleep. The descending signal for postural muscle atonia during REM sleep is thought to originate from glutamatergic neurons of the sublaterodorsal nucleus (SLD), which in turn activate glycinergic pre‐motor neurons in the spinal cord and/or ventromedial medulla to inhibit motor neurons. Despite work over the past two decades on many neurotransmitter systems that regulate the SLD, gaps remain in our knowledge of the synaptic basis by which SLD REM neurons are regulated and in turn produce REM sleep atonia. Elucidating the anatomical, cellular and synaptic basis of REM sleep atonia control is a critical step for treating many sleep‐related disorders including obstructive sleep apnoea (apnea), REM sleep behaviour disorder (RBD) and narcolepsy with cataplexy.

Neuroscience: A Distributed Neural Network Controls REM Sleep
How does the brain control dreams? New science shows that a small node of cells in the medulla — the most primitive part of the brain — may function to control REM sleep, the brain state that underlies dreaming.

Basal forebrain control of wakefulness and cortical rhythms
Wakefulness, along with fast cortical rhythms and associated cognition, depend on the basal forebrain (BF). BF cholinergic cell loss in dementia and the sedative effect of anti-cholinergic drugs have long implicated these neurons as important for cognition and wakefulness. The BF also contains intermingled inhibitory GABAergic and excitatory glutamatergic cell groups whose exact neurobiological roles are unclear. Here we show that genetically targeted chemogenetic activation of BF cholinergic or glutamatergic neurons in behaving mice produced significant effects on state consolidation and/or the electroencephalogram but had no effect on total wake. Similar activation of BF GABAergic neurons produced sustained wakefulness and high-frequency cortical rhythms, whereas chemogenetic inhibition increased sleep. Our findings reveal a major contribution of BF GABAergic neurons to wakefulness and the fast cortical rhythms associated with cognition. These findings may be clinically applicable to manipulations aimed at increasing forebrain activation in dementia and the minimally conscious state.
Targeted genetic manipulations of neuronal subtypes using promoter-specific combinatorial AAVs in wild-type animals
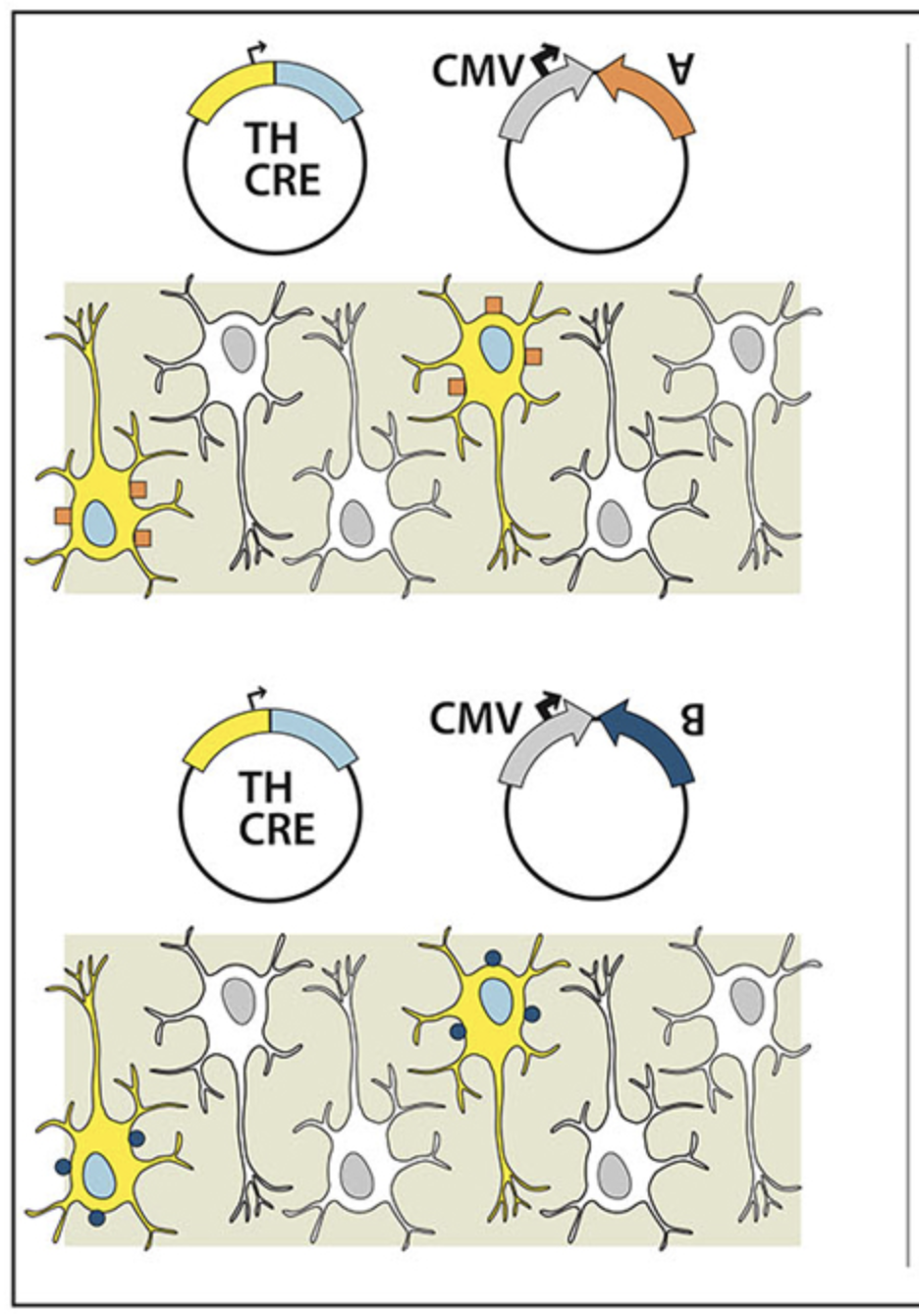
Techniques to genetically manipulate the activity of defined neuronal subpopulations have been useful in elucidating function, however applicability to translational research beyond transgenic mice is limited. Subtype targeted transgene expression can be achieved using specific promoters, but often currently available promoters are either too large to package into many vectors, in particular adeno-associated virus (AAV), or do not drive expression at levels sufficient to alter behavior. To permit neuron subtype specific gene expression in wildtype animals, we developed a combinatorial AAV targeting system that drives, in combination, subtype specific Cre-recombinase expression with a strong but non-specific Cre-conditional transgene. Using this system we demonstrate that the tyrosine hydroxylase promoter (TH-Cre-AAV) restricted expression of channelrhodopsin-2 (EF1α-DIO-ChR2-EYFP-AAV) to the rat ventral tegmental area (VTA), or an activating DREADD (hSyn-DIO-hM3Dq-mCherry-AAV) to the rat locus coeruleus (LC). High expression levels were achieved in both regions. Immunohistochemistry (IHC) showed the majority of ChR2+ neurons (>93%) colocalized with TH in the VTA, and optical stimulation evoked striatal dopamine release. Activation of TH neurons in the LC produced sustained EEG and behavioral arousal. TH-specific hM3Dq expression in the LC was further compared with: (1) a Cre construct driven by a strong but non-specific promoter (non-targeting); and (2) a retrogradely-transported WGA-Cre delivery mechanism (targeting a specific projection). IHC revealed that the area of c-fos activation after CNO treatment in the LC and peri-LC neurons appeared proportional to the resulting increase in wakefulness (non-targeted > targeted > ACC to LC projection restricted). Our dual AAV targeting system effectively overcomes the large size and weak activity barrier prevalent with many subtype specific promoters by functionally separating subtype specificity from promoter strength.
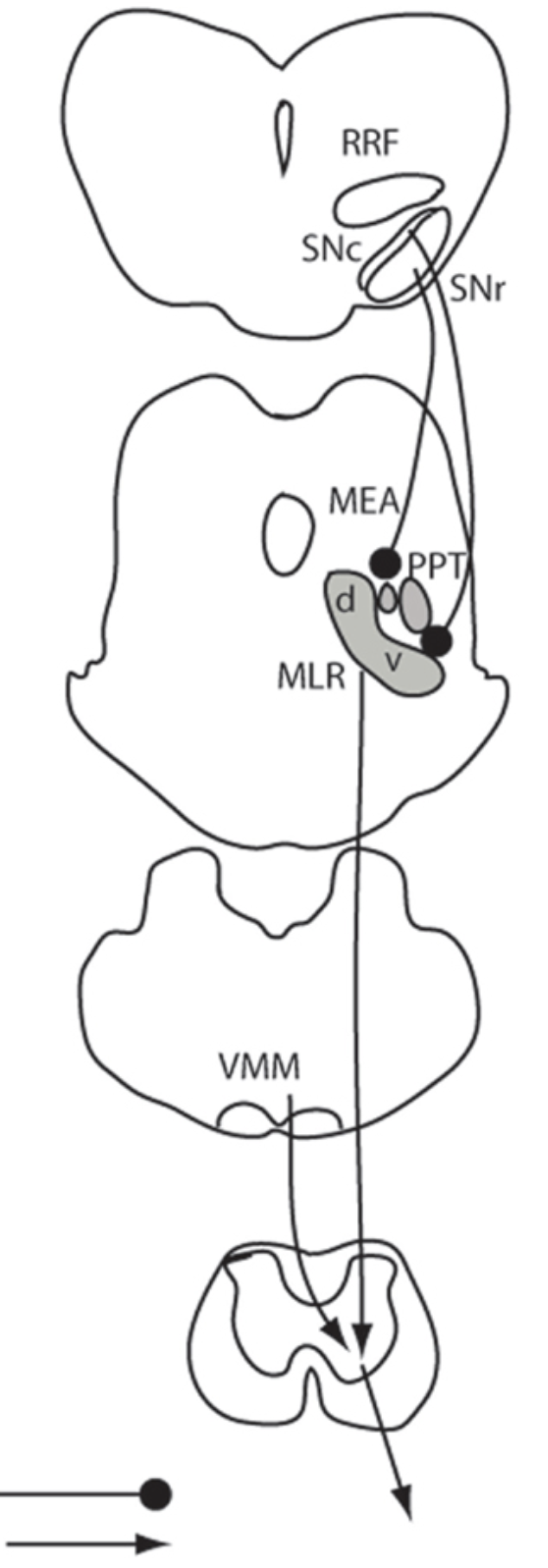
Anatomical location of the mesencephalic locomotor region and its possible role in locomotion, posture, cataplexy, and Parkinsonism
The mesencephalic (or midbrain) locomotor region (MLR) was first described in 1966 by Shik and colleagues, who demonstrated that electrical stimulation of this region induced locomotion in decerebrate (intercollicular transection) cats. The pedunculopontine tegmental nucleus (PPT) cholinergic neurons and midbrain extrapyramidal area (MEA) have been suggested to form the neuroanatomical basis for the MLR, but direct evidence for the role of these structures in locomotor behavior has been lacking. Here, we tested the hypothesis that the MLR is composed of non-cholinergic spinally projecting cells in the lateral pontine tegmentum. Our results showed that putative MLR neurons medial to the PPT and MEA in rats were non-cholinergic, glutamatergic, and express the orexin (hypocretin) type 2 receptors. Fos mapping correlated with motor behaviors revealed that the dorsal and ventral MLR are activated, respectively, in association with locomotion and an erect posture. Consistent with these findings, chemical stimulation of the dorsal MLR produced locomotion, whereas stimulation of the ventral MLR caused standing. Lesions of the MLR (dorsal and ventral regions together) resulted in cataplexy and episodic immobility of gait. Finally, trans-neuronal tracing with pseudorabies virus demonstrated disynaptic input to the MLR from the substantia nigra via the MEA. These findings offer a new perspective on the neuroanatomic basis of the MLR, and suggest that MLR dysfunction may contribute to the postural and gait abnormalities in Parkinsonism.

Medial Amygdalar Aromatase Neurons Regulate Aggression in Both Sexes
Aromatase-expressing neuroendocrine neurons in the vertebrate male brain synthesize estradiol from circulating testosterone. This locally produced estradiol controls neural circuits underlying courtship vocalization, mating, aggression, and territory marking in male mice. How aromatase-expressing neuronal populations control these diverse estrogen-dependent male behaviors is poorly understood, and the function, if any, of aromatase-expressing neurons in females is unclear. Using targeted genetic approaches, we show that aromatase-expressing neurons within the male posterodorsal medial amygdala (MeApd) regulate components of aggression, but not other estrogen-dependent male-typical behaviors. Remarkably, aromatase-expressing MeApd neurons in females are specifically required for components of maternal aggression, which we show is distinct from intermale aggression in pattern and execution. Thus, aromatase-expressing MeApd neurons control distinct forms of aggression in the two sexes. Moreover, our findings indicate that complex social behaviors are separable in a modular manner at the level of genetically identified neuronal populations.

Identification of a direct GABAergic pallidocortical pathway in rodents
The basal ganglia, interacting with the cortex, play a critical role in a range of behaviors. Output from the basal ganglia to the cortex is thought to relay through the thalamus, yet an intriguing alternative is that the basal ganglia may directly project to, and communicate with, the cortex. We explored an efferent projection from the globus pallidus externa (GPe), a key hub in the basal ganglia system, to the cortex of rats and mice. Anterograde and retrograde tracing revealed projections to the frontal premotor cortex, especially the deep projecting layers, originating from GPe neurons that receive axonal inputs from the dorsal striatum. Cre-dependent anterograde tracing in GPe Vgat-ires-cre mice confirmed that the pallidocortical projection is GABAergic, and in vitro optogenetic stimulation in the cortex of these projections produced a fast inhibitory postsynaptic current in targeted cells that was abolished by bicucculine. The pallidocortical projections targeted GABAergic interneurons and, to a lesser extent, pyramidal neurons. This GABAergic pallidocortical pathway directly links the basal ganglia and cortex and may play a key role in behavior and cognition in normal and disease states.
Impaired circadian photosensitivity in mice lacking glutamate transmission from retinal melanopsin cells
Work in animals and humans has suggested the existence of a slow wave sleep (SWS)-promoting/electroencephalogram (EEG)-synchronizing center in the mammalian lower brainstem. Although sleep-active GABAergic neurons in the medullary parafacial zone (PZ) are needed for normal SWS, it remains unclear whether these neurons can initiate and maintain SWS or EEG slow-wave activity (SWA) in behaving mice. We used genetically targeted activation and optogenetically based mapping to examine the downstream circuitry engaged by SWS-promoting PZ neurons, and we found that this circuit uniquely and potently initiated SWS and EEG SWA, regardless of the time of day. PZ neurons monosynaptically innervated and released synaptic GABA onto parabrachial neurons, which in turn projected to and released synaptic glutamate onto cortically projecting neurons of the magnocellular basal forebrain; thus, there is a circuit substrate through which GABAergic PZ neurons can potently trigger SWS and modulate the cortical EEG.n idea.
