Publications

Hypothalamic Pomc Neurons Innervate the Spinal Cord and Modulate the Excitability of Premotor Circuits
Locomotion requires energy, yet animals need to increase locomotion in order to find and consume food in energy-deprived states. While such energy homeostatic coordination suggests brain origin, whether the central melanocortin 4 receptor (Mc4r) system directly modulates locomotion through motor circuits is unknown. Here, we report that hypothalamic Pomc neurons in zebrafish and mice have long-range projections into spinal cord regions harboring Mc4r-expressing V2a interneurons, crucial components of the premotor networks. Furthermore, in zebrafish, Mc4r activation decreases the excitability of spinal V2a neurons as well as swimming and foraging, while systemic or V2a neuron-specific blockage of Mc4r promotes locomotion. In contrast, in mice, electrophysiological recordings revealed that two-thirds of V2a neurons in lamina X are excited by the Mc4r agonist α-MSH, and acute inhibition of Mc4r signaling reduces locomotor activity. In addition, we found other Mc4r neurons in spinal lamina X that are inhibited by α-MSH, which is in line with previous studies in rodents where Mc4r agonists reduced locomotor activity. Collectively, our studies identify spinal V2a interneurons as evolutionary conserved second-order neurons of the central Mc4r system, providing a direct anatomical and functional link between energy homeostasis and locomotor control systems. The net effects of this modulatory system on locomotor activity can vary between different vertebrate species and, possibly, even within one species. We discuss the biological sense of this phenomenon in light of the ambiguity of locomotion on energy balance and the different living conditions of the different species.

Study protocol for a randomised controlled trial evaluating the effects of the orexin receptor antagonist suvorexant on sleep architecture and delirium in the intensive care unit
Insomnia frequently occurs in patients admitted to an intensive care unit (ICU). Sleep-promoting agents may reduce rapid eye movement sleep and have deliriogenic effects. Suvorexant (Belsomra) is an orexin receptor antagonist with Food and Drug Administration (FDA) approval for the treatment of adult insomnia, which improves sleep onset and maintenance as well as subjective measures of quality of sleep. This trial will evaluate the efficacy of postoperative oral suvorexant treatment on night-time wakefulness after persistent sleep onset as well as the incidence and duration of delirium among adult cardiac surgical patients.

The neuroanatomy and neurochemistry of sleep-wake control
Sleep-wake control is dependent upon multiple brain areas widely distributed throughout the neural axis. Historically, the monoaminergic and cholinergic neurons of the ascending arousal system were the first to be discovered, and it was only relatively recently that GABAergic and glutamatergic wake- and sleep-promoting populations have been identified. Contemporary advances in molecular-genetic tools have revealed both the complexity and heterogeneity of GABAergic NREM sleep-promoting neurons as well as REM sleep-regulating populations in the brainstem such as glutamatergic neurons in the sublaterodorsal nucleus. The sleep-wake cycle progresses from periods of wakefulness to non-rapid eye movement (NREM) sleep and subsequently rapid eye movement (REM) sleep. Each vigilance stage is controlled by multiple neuronal populations, via a complex regulation that is still incompletely understood. In recent years the field has seen a proliferation in the identification and characterization of new neuronal populations involved in sleep-wake control thanks to newer, more powerful molecular genetic tools that are able to reveal neurophysiological functions via selective activation, inhibition and lesion of neuroanatomically defined sub-types of neurons that are widespread in the brain, such as GABAergic and glutamatergic neurons

Role of serotonergic dorsal raphe neurons in hypercapnia-induced arousals
During obstructive sleep apnea, elevation of CO2 during apneas contributes to awakening and restoring airway patency. We previously found that glutamatergic neurons in the external lateral parabrachial nucleus (PBel) containing calcitonin gene related peptide (PBelCGRP neurons) are critical for causing arousal during hypercapnia. However, others found that genetic deletion of serotonin (5HT) neurons in the brainstem also prevented arousal from hypercapnia. To examine interactions between the two systems, we showed that dorsal raphe (DR) 5HT neurons selectively targeted the PBel. Either genetically directed deletion or acute optogenetic silencing of DRSert neurons dramatically increased the latency of mice to arouse during hypercapnia, as did silencing DRSert terminals in the PBel. This effect was mediated by 5HT2a receptors which are expressed by PBelCGRP neurons. Our results indicate that the serotonergic input from the DR to the PBel via 5HT2a receptors is critical for modulating the sensitivity of the PBelCGRP neurons that cause arousal to rising levels of blood CO2.

An Inhibitory Lateral Hypothalamic-Preoptic Circuit Mediates Rapid Arousals from Sleep
Among the neuronal populations implicated in sleep-wake control, the ventrolateral preoptic (VLPO) nucleus has emerged as a key sleep-promoting center. However, the synaptic drives that regulate the VLPO to control arousal levels in vivo have not to date been identified. Here, we show that sleep-promoting galaninergic neurons within the VLPO nucleus, defined pharmacologically and by single-cell transcript analysis, are postsynaptic targets of lateral hypothalamic GABAergic (LHGABA) neurons and that activation of this pathway in vivo rapidly drives wakefulness. Ca2+ imaging from LHGABA neurons indicate that they are both wake and rapid eye movement (REM)-sleep active. Consistent with the potent arousal-promoting property of the LHGABA → VLPO pathway, presynaptic inputs to LHGABA neurons originate from several canonical stress- and arousal-related network nodes. This work represents the first demonstration that direct synaptic inhibition of the VLPO area can suppress sleep-promoting neurons to rapidly promote arousal.

Selective activation of serotoninergic dorsal raphe neurons facilitates sleep through anxiolysis
A role for the brain’s serotoninergic (5HT) system in the regulation of sleep and wakefulness has been long suggested. Yet, previous studies employing pharmacological, lesion and genetically driven approaches have produced inconsistent findings, leaving 5HT’s role in sleep-wake regulation incompletely understood. Here we sought to define the specific contribution of 5HT neurons within the dorsal raphe nucleus (DRN5HT) to sleep and arousal control. To do this, we employed a chemogenetic strategy to selectively and acutely activate DRN5HT neurons and monitored sleep-wake using electroencephalogram recordings. We additionally assessed indices of anxiety using the open field and elevated plus maze behavioral tests and employed telemetric-based recordings to test effects of acute DRN5HT activation on body temperature and locomotor activity. Our findings indicate that the DRN5HT cell population may not modulate sleep-wake per se, but rather that its activation has apparent anxiolytic properties, suggesting the more nuanced view that DRN5HT neurons are sleep permissive under circumstances that produce anxiety or stress.
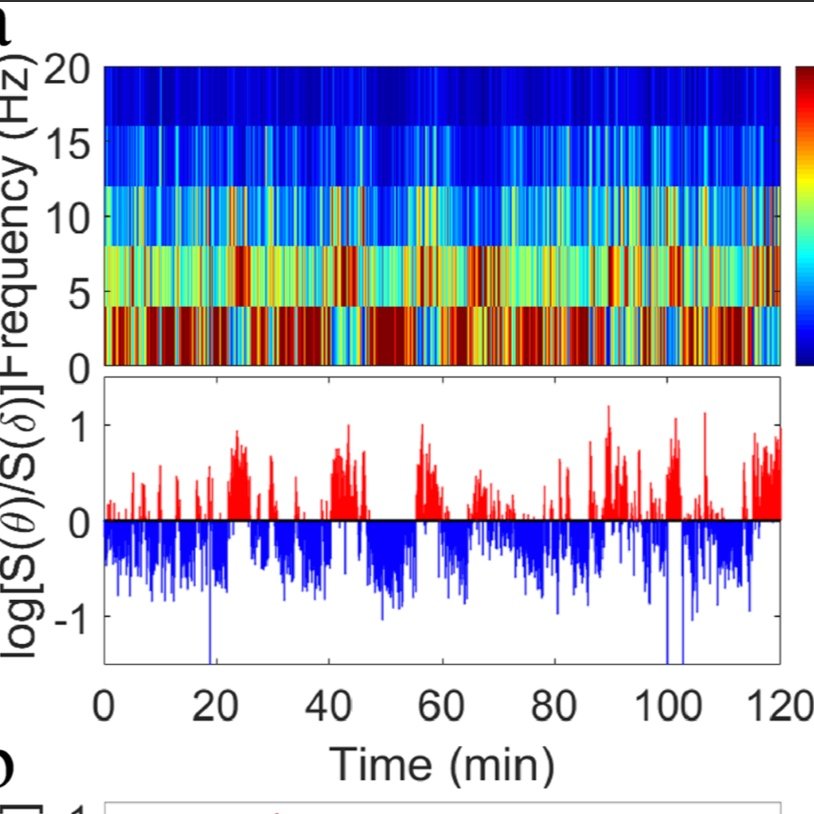
Non-equilibrium critical dynamics of bursts in θ and δrhythms as fundamental characteristic of sleep and wake micro-architecture
Origin and functions of intermittent transitions among sleep stages, including short awakenings and arousals, constitute a challenge to the current homeostatic framework for sleep regulation, focusing on factors modulating sleep over large time scales. Here we propose that the complex micro-architecture characterizing the sleep-wake cycle results from an underlying non-equilibrium critical dynamics, bridging collective behaviors across spatio-temporal scales. We investigate θ and δ wave dynamics in control rats and in rats with lesions of sleep-promoting neurons in the parafacial zone. We demonstrate that intermittent bursts in θ and δ rhythms exhibit a complex temporal organization, with long-range power-law correlations and a robust duality of power law (θ-bursts, active phase) and exponential-like (δ-bursts, quiescent phase) duration distributions, typical features of non-equilibrium systems self-organizing at criticality. Crucially, such temporal organization relates to anti-correlated coupling between θ- and δ-bursts, and is independent of the dominant physiologic state and lesions, a solid indication of a basic principle in sleep dynamics.

Reassessing the Role of Histaminergic Tuberomammillary Neurons in Arousal Control
The histaminergic neurons of the tuberomammillary nucleus (TMNHDC) of the posterior hypothalamus have long been implicated in promoting arousal. More recently, a role for GABAergic signaling by the TMNHDC neurons in arousal control has been proposed. Here, we investigated the effects of selective chronic disruption of GABA synthesis (via genetic deletion of the GABA synthesis enzyme, glutamic acid decarboxylase 67) or GABAergic transmission (via genetic deletion of the vesicular GABA transporter (VGAT)) in the TMNHDC neurons on sleep–wake in male mice. We also examined the effects of acute chemogenetic activation and optogenetic inhibition of TMNHDC neurons upon arousal in male mice. Unexpectedly, we found that neither disruption of GABA synthesis nor GABAergic transmission altered hourly sleep–wake quantities, perhaps because very few TMNHDC neurons coexpressed VGAT. Acute chemogenetic activation of TMNHDC neurons did not increase arousal levels above baseline but did enhance vigilance when the mice were exposed to a behavioral cage change challenge. Similarly, acute optogenetic inhibition had little effect upon baseline levels of arousal. In conclusion, we could not identify a role for GABA release by TMNHDC neurons in arousal control. Further, if TMNHDC neurons do release GABA, the mechanism by which they do so remains unclear. Our findings support the view that TMNHDC neurons may be important for enhancing arousal under certain conditions, such as exposure to a novel environment, but play only a minor role in behavioral and EEG arousal under baseline conditions.

The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans
The sleep-wake cycle regulates interstitial fluid (ISF) and cerebrospinal (CSF) levels of amyloid-β (Aβ) that accumulates in Alzheimer disease (AD) and chronic sleep deprivation (SD) increases Aβ plaques. However, tau not Aβ accumulation appears to drive AD neurodegeneration. Therefore, we tested whether ISF/CSF tau and tau seeding/spreading was influenced by the sleep-wake cycle and SD. Mouse ISF tau was increased ~90% during normal wakefulness vs. sleep and ~100% during SD. Human CSF tau also increased over 50% during SD. In a tau seeding and spreading model, chronic SD increased tau pathology spreading. Chemogenetically-driven wakefulness in mice also significantly increased both ISF Aβ and tau. Thus, the sleep-wake cycle regulates ISF tau and sleep deprivation increases ISF and CSF tau as well as tau pathology spreading.

To eat or to sleep: That is a lateral hypothalamic question
The lateral hypothalamus (LH) is a functionally and anatomically complex brain region that is involved in the regulation of many behavioral and physiological processes including feeding, arousal, energy balance, stress, reward and motivated behaviors, pain perception, body temperature regulation, digestive functions and blood pressure. Despite noteworthy experimental efforts over the past decades, the circuit, cellular and synaptic bases by which these different processes are regulated by the LH remains incompletely understood. This knowledge gap links in large part to the high cellular heterogeneity of the LH. Fortunately, the rapid evolution of newer genetic and electrophysiological tools is now permitting the selective manipulation, typically genetically-driven, of discrete LH cell populations. This, in turn, permits not only assignment of function to discrete cell groups, but also reveals that considerable synergistic and antagonistic interactions exist between key LH cell populations that regulate feeding and arousal. For example, we now know that while LH melanin-concentrating hormone (MCH) and orexin/hypocretin neurons both function as sensors of the internal metabolic environment, their roles regulating sleep and arousal are actually opposing. Additional studies have uncovered similarly important roles for subpopulations of LH GABAergic cells in the regulation of both feeding and arousal. Herein we review the role of LH MCH, orexin/hypocretin and GABAergic cell populations in the regulation of energy homeostasis (including feeding) and sleep-wake and discuss how these three cell populations, and their subpopulations, may interact to optimize and coordinate metabolism, sleep and arousal

Differential Role of Pontomedullary Glutamatergic Neuronal Populations in Sleep-Wake Control
Parafacial zone (PZ) GABAergic neurons play a major role in slow-wave-sleep (SWS), also called non-rapid eye movement (NREM) sleep. The PZ also contains glutamatergic neurons expressing the vesicular transporter for glutamate, isoform 2 (Vglut2). We hypothesized that PZ Vglut2-expressing (PZVglut2) neurons are also involved in sleep control, playing a synergistic role with PZ GABAergic neurons. To test this hypothesis, we specifically activated PZVglut2 neurons using the excitatory chemogenetic receptor hM3Dq. Anatomical inspection of the injection sites revealed hM3Dq transfection in PZ, parabrachial nucleus (PB), sublaterodorsal nucleus (SLD) or various combinations of these three brain areas. Consistent with the known wake- and REM sleep-promoting role of PB and SLD, respectively, chemogenetic activation of PBVglut2 or SLDVglut2 resulted in wake or REM sleep enhancement. Chemogenetic activation of PZVglut2 neurons did not affect sleep-wake phenotype during the mouse active period but increased wakefulness and REM sleep, similar to PBVglut2 and SLDVglut2 activation, during the rest period. To definitively confirm the role of PZVglut2 neurons, we used a specific marker for PZVglut2 neurons, Phox2B. Chemogenetic activation of PZPhox2B neurons did not affect sleep-wake phenotype, indicating that PZ glutamatergic neurons are not sufficient to affect sleep-wake cycle. These results indicate that PZ glutamatergic neurons are not involved in sleep-wake control.

Newly identified sleep–wake and circadian circuits as potential therapeutic targets
Optogenetics and chemogenetics are powerful tools, allowing the specific activation or inhibition of targeted neuronal subpopulations. Application of these techniques to sleep and circadian research has resulted in the unveiling of several neuronal populations that are involved in sleep–wake control, and allowed a comprehensive interrogation of the circuitry through which these nodes are coordinated to orchestrate the sleep–wake cycle. In this review, we discuss six recently described sleep–wake and circadian circuits that show promise as therapeutic targets for sleep medicine. The parafacial zone (PZ) and the ventral tegmental area (VTA) are potential druggable targets for the treatment of insomnia. The brainstem circuit underlying rapid eye movement sleep behavior disorder (RBD) offers new possibilities for treating RBD and neurodegenerative synucleinopathies, whereas the parabrachial nucleus, as a nexus linking arousal state control and breathing, is a promising target for developing treatments for sleep apnea. Therapies that act upon the hypothalamic circuitry underlying the circadian regulation of aggression or the photic regulation of arousal and mood pathway carry enormous potential for helping to reduce the socioeconomic burden of neuropsychiatric and neurodegenerative disorders on society. Intriguingly, the development of chemogenetics as a therapeutic strategy is now well underway and such an approach has the capacity to lead to more focused and less invasive therapies for treating sleep–wake disorders and related comorbidities.

A Glutamatergic Hypothalamomedullary Circuit Mediates Thermogenesis, but Not Heat Conservation, during Stress-Induced Hyperthermia
Stress elicits a variety of autonomic responses, including hyperthermia (stress fever) in humans and animals. In this present study, we investigated the circuit basis for thermogenesis and heat conservation during this response. We first demonstrated the glutamatergic identity of the dorsal hypothalamic area (DHAVglut2) neurons that innervate the raphe pallidus nucleus (RPa) to regulate core temperature (Tc) and mediate stress-induced hyperthermia. Then, using chemogenetic and optogenetic methods to manipulate this hypothalamomedullary circuit, we found that activation of DHAVglut2 neurons potently drove an increase in Tc, but surprisingly, stress-induced hyperthermia was only reduced by about one-third when they were inhibited. Further investigation showed that DHAVglut2 neurons activate brown adipose tissue (BAT) but do not cause vasoconstriction, instead allowing reflex tail artery vasodilation as a response to BAT-induced hyperthermia. Retrograde rabies virus tracing revealed projections from DHAVglut2 neurons to RPaVglut3, but not to RPaGABA neurons, and identified a set of inputs to DHAVglut2 → RPa neurons that are likely to mediate BAT activation. The dissociation of the DHAVglut2 thermogenic pathway from the thermoregulatory vasoconstriction (heat-conserving) pathway may explain stress flushing (skin vasodilation but a feeling of being too hot) during stressful times.

Lateral Hypothalamic Area Neurotensin Neurons Are Required for Control of Orexin Neurons and Energy Balance
The lateral hypothalamic area (LHA) is essential for motivated ingestive and locomotor behaviors that impact body weight, yet it remains unclear how the neurochemically defined subpopulations of LHA neurons contribute to energy balance. In particular, the role of the large population of LHA neurotensin (Nts) neurons has remained ambiguous due to the lack of methods to easily visualize and modulate these neurons. Because LHA Nts neurons are activated by leptin and other anorectic cues and they modulate dopamine or local LHA orexin neurons implicated in energy balance, they may have important, unappreciated roles for coordinating behaviors necessary for proper body weight. In this study, we genetically ablated or chemogenetically inhibited LHA Nts neurons in adult mice to determine their necessity for control of motivated behaviors and body weight. Genetic ablation of LHA Nts neurons resulted in profoundly increased adiposity compared with mice with intact LHA Nts neurons, as well as diminished locomotor activity, energy expenditure, and water intake. Complete loss of LHA Nts neurons also led to downregulation of orexin, revealing important cross-talk between the LHA Nts and orexin populations in maintenance of behavior and body weight. In contrast, chemogenetic inhibition of intact LHA Nts neurons did not disrupt orexin expression, but it suppressed locomotor activity and the adaptive response to leptin. Taken together, these data reveal the necessity of LHA Nts neurons and their activation for controlling energy balance, and that LHA Nts neurons influence behavior and body weight via orexin-dependent and orexin-independent mechanisms.

Functionally Complete Excision of Conditional Alleles in the Mouse Suprachiasmatic Nucleus by Vgat-ires-Cre
Mice with targeted gene disruption have provided important information about the molecular mechanisms of circadian clock function. A full understanding of the roles of circadian-relevant genes requires manipulation of their expression in a tissue-specific manner, ideally including manipulation with high efficiency within the suprachiasmatic nuclei (SCN). To date, conditional manipulation of genes within the SCN has been difficult. In a previously developed mouse line, Cre recombinase was inserted into the vesicular GABA transporter (Vgat) locus. Since virtually all SCN neurons are GABAergic, this Vgat-Cre line seemed likely to have high efficiency at disrupting conditional alleles in SCN. To test this premise, the efficacy of Vgat-Cre in excising conditional (fl, for flanked by LoxP) alleles in the SCN was examined. Vgat-Cre-mediated excision of conditional alleles of Clock or Bmal1 led to loss of immunostaining for products of the targeted genes in the SCN. Vgat-Cre+; Clockfl/fl; Npas2m/m mice and Vgat-Cre+; Bmal1fl/fl mice became arrhythmic immediately upon exposure to constant darkness, as expected based on the phenotype of mice in which these genes are disrupted throughout the body. The phenotype of mice with other combinations of Vgat-Cre+, conditional Clock, and mutant Npas2 alleles also resembled the corresponding whole-body knockout mice. These data indicate that the Vgat-Cre line is useful for Cre-mediated recombination within the SCN, making it useful for Cre-enabled technologies including gene disruption, gene replacement, and opto- and chemogenetic manipulation of the SCN circadian clock.

Genetic Activation, Inactivation, and Deletion Reveal a Limited And Nuanced Role for Somatostatin-Containing Basal Forebrain Neurons in Behavioral State Control
Recent studies have identified an especially important role for basal forebrain GABAergic (BFVGAT) neurons in the regulation of behavioral waking and fast cortical rhythms associated with cognition. However, BFVGAT neurons comprise several neurochemically and anatomically distinct subpopulations, including parvalbumin-containing BFVGAT neurons and somatostatin-containing BFVGAT neurons (BFSOM neurons), and it was recently reported that optogenetic activation of BFSOM neurons increases the probability of a wakefulness to non-rapid-eye movement (NREM) sleep transition when stimulated during the rest period of the animal. This finding was unexpected given that most BFSOM neurons are not NREM sleep active and that central administration of the synthetic somatostatin analog, octreotide, suppresses NREM sleep or increases REM sleep. Here we used a combination of genetically driven chemogenetic and optogenetic activation, chemogenetic inhibition, and ablation approaches to further explore the in vivo role of BFSOM neurons in arousal control. Our findings indicate that acute activation or inhibition of BFSOM neurons is neither wakefulness nor NREM sleep promoting and is without significant effect on the EEG, and that chronic loss of these neurons is without effect on total 24 h sleep amounts, although a small but significant increase in waking was observed in the lesioned mice during the early active period. Our in vitro cell recordings further reveal electrophysiological heterogeneity in BFSOM neurons, specifically suggesting at least two distinct subpopulations. Together, our data support the more nuanced view that BFSOM neurons are electrically heterogeneous and are not NREM sleep or wake promoting per se, but may exert, in particular during the early active period, a modest inhibitory influence on arousal circuitry.

A hypothalamic circuit for the circadian control of aggression
“Sundowning” in dementia and Alzheimer’s disease is characterized by early evening agitation and aggression. While such periodicity suggests a circadian origin, whether the circadian clock directly regulates aggressive behavior is unknown. We demonstrate that a daily rhythm in aggression propensity in male mice is gated by GABAergic subparaventricular zone (SPZGABA) neurons, the major postsynaptic targets of the central circadian clock, the suprachiasmatic nucleus (SCN). Optogenetic mapping revealed that SPZGABA neurons receive input from vasoactive intestinal polypeptide SCN neurons and innervate neurons in the ventrolateral part of the ventromedial hypothalamus (VMHvl) known to regulate aggression. Additionally, VMH-projecting dorsal SPZ neurons are more active during early day than early night, and acute chemogenetic inhibition of SPZGABA transmission phase-dependently increases aggression. Finally, SPZGABA-recipient central VMH neurons directly innervate VMHvl neurons and activation of this intra-VMH circuit drove attack behavior. Altogether, we reveal a functional polysynaptic circuit by which the SCN clock regulates aggression.

Hippocampal corticotropin-releasing hormone neurons support recognition memory and modulate hippocampal excitability
Corticotropin-releasing hormone (CRH) signaling in the hippocampus has been established to be important for mediating the effects of stress on learning and memory. Given our laboratory’s recent characterization of a subset of hippocampal CRH neurons as a novel class of GABAergic interneurons, we hypothesized that these local GABAergic hippocampal CRH neurons may influence hippocampal function. Here we applied an array of molecular tools to selectively label and manipulate hippocampal CRH neurons in mice, in order to assess this interneuron population’s impact on hippocampus-dependent behaviors and hippocampal network excitability. Genetically-targeted ablation of hippocampal CRH neurons in vivo impaired object recognition memory and substantially enhanced the severity of kainic acid-induced seizures. Conversely, selective activation of CRH neurons in vitro suppressed the excitability of the mossy fiber-CA3 pathway. Additional experiments are needed to reconcile the functions of GABA and CRH signaling of hippocampal CRH neurons on hippocampal function. However, our results indicate that this interneuron population plays an important role in maintaining adaptive network excitability, and provide a specific circuit-level mechanism for this role.

The Biology of REM Sleep
Considerable advances in our understanding of the mechanisms and functions of rapid-eye-movement (REM) sleep have occurred over the past decade. Much of this progress can be attributed to the development of new neuroscience tools that have enabled high-precision interrogation of brain circuitry linked with REM sleep control, in turn revealing how REM sleep mechanisms themselves impact processes such as sensorimotor function. This review is intended to update the general scientific community about the recent mechanistic, functional and conceptual developments in our current understanding of REM sleep biology and pathobiology. Specifically, this review outlines the historical origins of the discovery of REM sleep, the diversity of REM sleep expression across and within species, the potential functions of REM sleep (e.g., memory consolidation), the neural circuits that control REM sleep, and how dysfunction of REM sleep mechanisms underlie debilitating sleep disorders such as REM sleep behaviour disorder and narcolepsy.

Supramammillary glutamate neurons are a key node of the arousal system
Basic and clinical observations suggest that the caudal hypothalamus comprises a key node of the ascending arousal system, but the cell types underlying this are not fully understood. Here we report that glutamate-releasing neurons of the supramammillary region (SuMvglut2) produce sustained behavioral and EEG arousal when chemogenetically activated. This effect is nearly abolished following selective genetic disruption of glutamate release from SuMvglut2 neurons. Inhibition of SuMvglut2 neurons decreases and fragments wake, also suppressing theta and gamma frequency EEG activity. SuMvglut2 neurons include a subpopulation containing both glutamate and GABA (SuMvgat/vglut2) and another also expressing nitric oxide synthase (SuMNos1/Vglut2). Activation of SuMvgat/vglut2 neurons produces minimal wake and optogenetic stimulation of SuMvgat/vglut2 terminals elicits monosynaptic release of both glutamate and GABA onto dentate granule cells. Activation of SuMNos1/Vglut2 neurons potently drives wakefulness, whereas inhibition reduces REM sleep theta activity. These results identify SuMvglut2 neurons as a key node of the wake−sleep regulatory system.
